The de novo DNA methyltransferase DNMT3A is important for normal hematopoiesis and hematopoietic stem cell (HSC) differentiation. Mutations in DNMT3A in humans lead to clonal expansion and a higher risk of malignancy development. In mice, ablation of DNMT3A enhances self-renewal and inhibits differentiation. Given that the pool of HSCs is heterogeneous, we considered whether DNMT3A loss preferentially affected particular HSC sub-types and hypothesized a role in megakaryocyte (Mk)-primed HSCs, given their position at the very top of the HSC hierarchy.
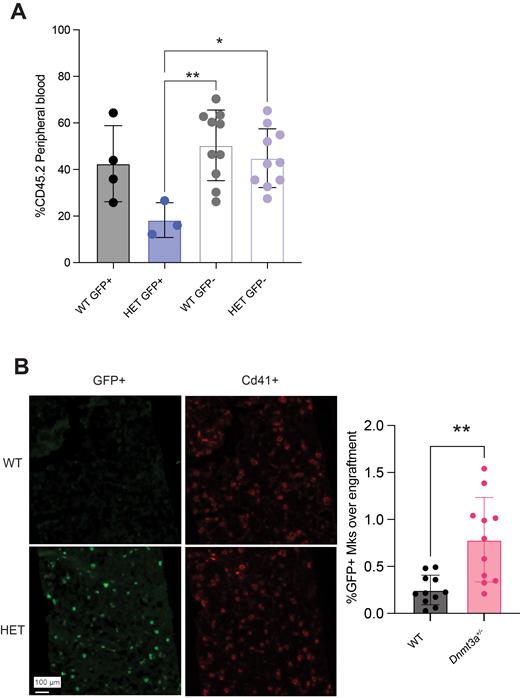
To examine this, we utilized Dnmt3a+/- mice in which Mk-primed HSCs are labeled with GFP due to an insertion of GFP under the von Willebrand factor (VWF) promoter (Sanjuan-Pla et al. 2013, Nature). This strain also facilitates the tracing of GFP pos megakaryocytes (Mks) and platelets in Dnmt3a+/- mice. We performed competitive transplantation with HSCs that were either GFP pos (Mk-primed) or GFP neg from WT and Dnmt3a+/- mice. The Dnmt3a+/- GFP pos HSCs exhibited significantly lower engraftment than the GFP neg conditions at 6 weeks post-transplant (Fig. A). While Mk-primed HSCs exhibited delayed lymphoid contribution, which was exacerbated in the Dnmt3a+/- condition, lymphoid contribution normalized in all groups by 18 weeks post-transplant. Strikingly, the Dnmt3a+/- GFP pos HSCs displayed significantly higher platelet contribution than all other groups relative to their engraftment without altering total platelet counts.
We also performed competitive transplantation with WT or Dnmt3a+/- GFP pos whole bone marrow. Upon secondary transplant, the Dnmt3a+/- cells exhibited significantly higher overall contribution to blood production. Additionally, the Dnmt3a+/- bone marrow displayed a marked myeloid/Mk/platelet bias relative to engraftment (Fig. B), and a significantly higher percentage of the HSC compartment was Mk-primed, although the total progenitor pool was smaller. It is likely that the enhanced number of Mk-primed HSCs in the Dnmt3a+/- secondary transplant condition contributes to increased myeloid/Mk/platelet skewing but that overall engraftment is rescued by the presence of GFP neg HSCs.
We next examined whether the differences observed in the HSC vs whole bone marrow transplant models were due to transplant stress or were present in the germline Dnmt3a+/- animals. Although we observed a slight increase in the number of Mks in the bone marrow in the germline Dnmt3a+/- animals, their Mks were the same size as WT. Their Mk ploidy, megakaryocyte-erythroid progenitor (MEP) composition, and %GFP pos HSCs were similar to littermate controls. However, while WT GFP pos HSCs were exceptionally enriched in the portion of HSCs with the highest stemness potential (marked by high Hoechst efflux and high CD150 expression), this enrichment was abrogated in Dnmt3a+/- GFP pos HSCs. Overall, these data indicate that megakaryocyte and platelet production is augmented from Dnmt3a+/- HSCs, likely due to shifts in the composition of the HSC pool, which are exacerbated after transplantation.
To determine whether DNMT3A loss affects Mk and platelet function, we cultured bone-marrow-derived Mks and isolated platelets from mouse blood. Dnmt3a+/- Mks formed proplatelets at similar rates to WT. Dnmt3a+/- platelets activated and aggregated in response to agonists, although at the gene and protein expression levels, several coagulation factors and key cytoskeletal components were downregulated. Finally, we subjected WT and Dnmt3a+/- MEPs and Mks to enzymatic DNA methyl-sequencing to examine how genome-wide methylation patterns are altered during the differentiation trajectory in the WT and Dnmt3a+/- contexts.
Our findings demonstrate that DNMT3A plays an important role in the lineage fate decisions of Mk-primed HSCs and implicates DNA methylation in Mk differentiation. Our data suggest that platelets generated from DNMT3A-mutant Mks may be over-represented in the blood of individuals with clonal hematopoiesis (CH) relative to apparent clone size. Given the association between CH and increased risk of cardiovascular disease and stroke, the role of DNMT3A-loss in Mk and platelet biogenesis and function warrants further investigation.
Disclosures
Rau:Servier Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal